Sounds disappointing, right? One cookie is full of chocolate chips in every bite, the next one’s almost bare. Now swap cookies for medicine. If one tablet has too much active ingredient and the next barely any? That’s not just disappointing—it’s dangerous.
That’s where the content uniformity of tablets comes in.
Whether you’re new to tablet manufacturing or a seasoned pro brushing up on quality control, this post breaks down tablet content uniformity in the simplest way possible—no jargon, no stress. Just clear, practical info you can use right away.
Let’s dig in.
What Is Content Uniformity of Tablets?
You don’t need to get too caught up in this jargon. Just think about chocolate chip cookies again. We all want every cookie to have a fair share of chips. That’s exactly the goal in tablet making. But instead of chocolate chips, it’s the active ingredient that needs to be spread out evenly.

This is what content uniformity is all about. It means making sure each tablet in a batch has pretty much the same amount of medicine.
Why Is Content Uniformity in Tablets So Important?
Content uniformity of tablets isn’t just some technical buzzword. It’s one of the most critical quality factors in pharmaceutical manufacturing. Here’s why it deserves your full attention:
1. Accurate Dosing
This is the heart of it. Patients have to get the right amount of medicine every single time they take a dose.

If the active ingredient in the tablet is unevenly distributed:
- Too much API: You risk overdosing, side effects, or even toxicity.
- Too little API: The treatment may not work at all.
Plus, many patients split tablets. Sometimes to adjust the dose, sometimes just to save money.
But here’s the thing: if the drug isn’t mixed evenly, a half tablet might not be half a dose. Uniform content makes sure that even halves (or quarters) of a tablet have the right amount of API in them.
2. Regulatory Compliance
Content uniformity of tablets is mandated by pharmacopeias (USP <905>, Ph. Eur., etc.).
If you fail to meet their standards, you might end up with:
- Batch rejection
- Product recalls
- Warning letters
- Potential shutdowns or license suspensions
3. Fewer Recalls
Recalls are expensive and time-consuming. They also erode customer trust and damage your brand image.
Most drug recalls happen because of dosage inconsistency. And this traces right back to poor tablet content uniformity.
4. Trust Building
When doctors prescribe your medication, they’re putting their trust in your product. Patients are trusting you every time they take a pill.

Delivering a consistent dose every single time isn’t just a regulatory requirement—it’s a promise you make to the people depending on your tablets.
What Causes Poor Tablet Content Uniformity?
| Category | Cause | What It Means |
| Formulation-related | Big particle size differences | Ingredients separate instead of mixing evenly. |
| Density mismatch | Heavy particles sink, light ones rise, causing separation. | |
| Sticky or static powders | Powders clump or stick, leading to uneven mixing. | |
| Low-dose tablet | Tiny amounts of medicine are harder to spread evenly. | |
| Moisture issues | High humidity leads to clumping, while low humidity causes static. | |
| Process-related | Under-mixing | Not enough blending time leaves the drug unevenly spread. |
| Over-mixing | Mixing too long can make particles separate again. | |
| Segregation during transfer | Powders can separate while moving after mixing. | |
| Poor granulation | Uneven granules lead to tablets with varying drug amounts. | |
| Uneven die filling | Poor powder flow results in weight and dose variation. | |
| Equipment-related | Wrong or overloaded blender | Can’t mix well if the blender is the wrong size or overfilled. |
| Bad feeder/hopper design | Poor design or vibration causes ingredients to separate. | |
| Incorrect press settings | Bad settings lead to uneven powder fill in tablets. | |
| Worn tooling or poor calibration | Old or misaligned parts cause dosing errors. |
How Is Tablet Content Uniformity Measured?
You might be wondering: how do we actually test content uniformity in a batch of tablets? As a rule of thumb, if a tablet has less than 25 mg of drug, or the drug is less than 25% of the tablet’s weight, it must go through a content uniformity test.
Content Uniformity Test for Tablets: The USP <905> Method
This is the most common industry standard. Here’s how it works:
- Step 1: Pick 10 tablets randomly from a batch.
- Step 2: Measure the amount of API in each pill.
- Step 3: Compare the results against the label claim (the target amount of API).
- Step 4: If all 10 tablets fall within 85-115% of the target, you pass.
- Step 5: If 1-3 tablets fall slightly outside this range (but not too far), you test 20 more tablets.
Fail: If any tablet is way off (outside 75-125%) or too many fall outside 85-115%.
Note: These ranges may differ depending on the pharmacopeia you’re following (USP, EP, JP, etc.), but the idea is the same.
(Side note: Some newer regulations even discuss testing 100 or more tablets using rapid spectroscopic methods. But in practice, USP <905> content uniformity with 10 (or 30) units is still the standard approach.)
Tools and Techniques to Improve the Content Uniformity of Tablets
If you’re struggling with uniformity and don’t know what to do, here are some secret weapons we’d like to share.
1. Use the Right Blending Equipment
If the API isn’t evenly mixed into the powder from the very beginning, you might not get a uniform drug content. So, all you need is a piece of reliable blending equipment.
Choosing the right blender depends on your material and batch size:
- V Blender machine or Bin Blender for general use

- High-shear mixers for more thorough blending

2. Try Granulation Techniques
If you’re handling low-dose drugs, the chance is that segregation will happen because these drugs only contain a tiny amount of the API. In this case, you need to use a granulator machine.
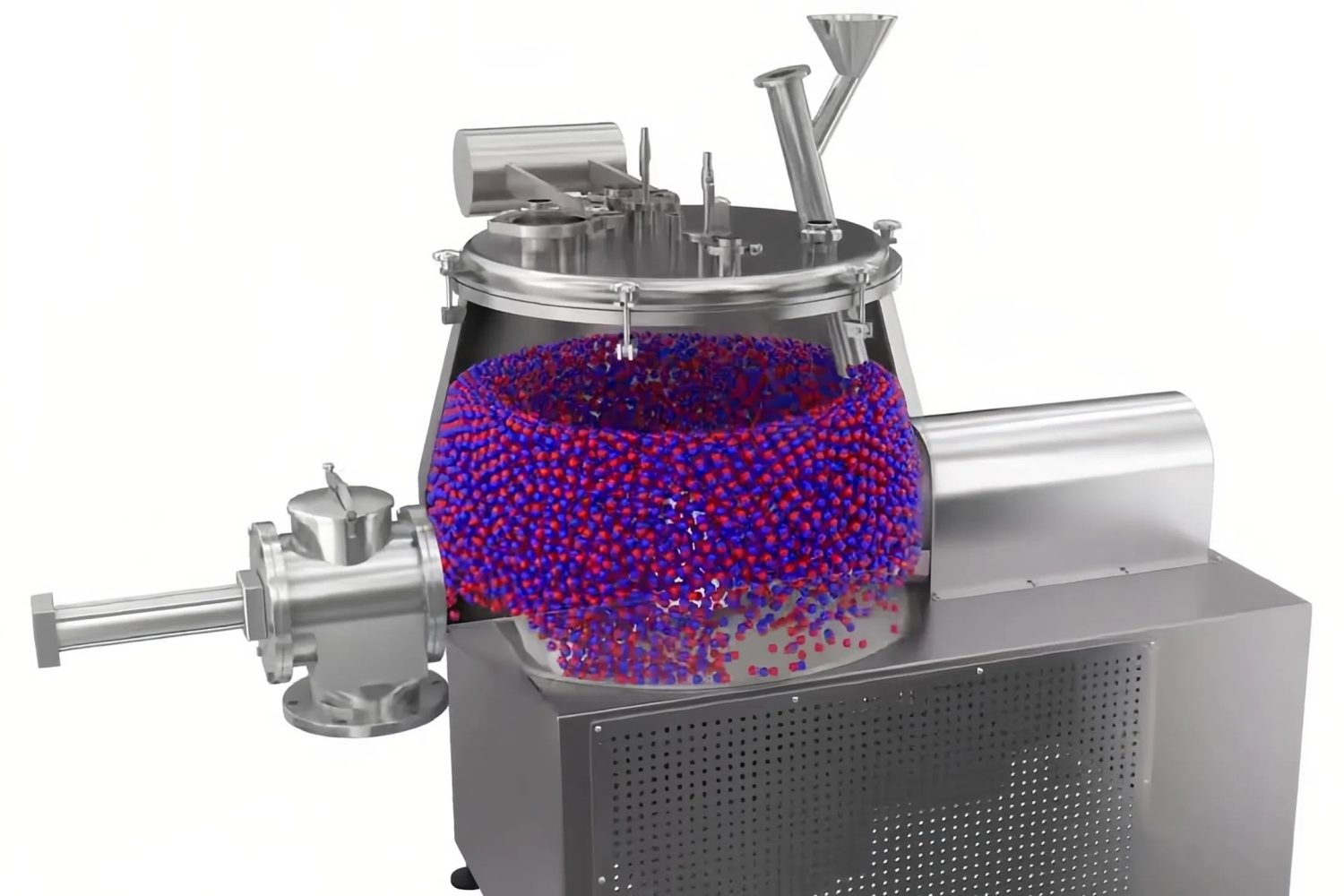
There are two approaches that can help ensure each granule has a uniform medicine distribution. They are:
- Wet Granulation: Uses a liquid binder to stick the particles together for better distribution.
- Dry Granulation: Useful for moisture-sensitive APIs.
3. Optimize Powder Flow
Poor powder flow = uneven filling = non-uniform tablets.
Solutions include:
- Adding flow agents like magnesium stearate
- Using equipment like tablet presses with forced feeders
- Monitoring granule size distribution
4. Control Tablet Press Settings
Finally, you get a perfect powder blend. But it’s still not a one-and-done solution. Your tablet press machine settings must be tightly controlled.

If you use an automated rotary press, pay attention to these parameters:
- Compression force
- Fill depth
- Press speed
How to Troubleshoot Tablet Content Uniformity Issues
Still getting bad results? Don’t panic—it happens. Here’s how you can troubleshoot uniformity problems:
Quick Fix Tip 1: Audit your blending process
- Adjust the blending time or switch to a different blender type. Let’s say you’re using the V-blender, try a bin blender.
- Take the blend samples from its top, middle, and bottom.
Quick Fix Tip 2: Check your API distribution before compression
- Take random pre-compression samples from different containers or hoppers to confirm API consistency.
Quick Fix Tip 3: Examine your equipment
- Regularly inspect and maintain your tablet press
- Check tooling alignment
- Clean the die cavity
- Replace worn parts
FAQs About Content Uniformity of Tablets
Q1. What’s the best way to measure if a dose is uniform?
A: There are two ways to check: content uniformity and weight variation.
Q2. What’s the difference between content uniformity and weight variation?
A: Weight variation checks if tablets weigh the same. Content uniformity checks if each one has the same amount of API. You can have a consistent weight and still fail a content test.
Q3. Do all tablets need a content uniformity test?
A: No, but most do. If the API is over a certain percentage of the tablet’s weight (typically >25%), a weight variation test may be enough. But for low-dose tablets, content uniformity tests are required.
Q4. What equipment helps improve content uniformity?
A: Blenders, granulators, and high-precision tablet presses with uniform die filling mechanisms are all essential.
Q5. How often do I have to check the content uniformity of tablets?
A: Typically per batch. But the exact frequency will depend on what the regulators say.
Final Word
Content uniformity of tablets is about making safe, effective medicine that people can trust. Poor uniformity can hurt your brand and, most importantly, your customers.
Whether you’re just setting up your tablet production line or refining your current process, always keep uniformity top of mind. Mix well. Measure smart. And test, test, test.
If you need help choosing the right blending or compression equipment to improve your tablet content uniformity, we’re here to help!