If you import a tablet press or capsule filler to the United States or the EU, there are some regulations you must know. They not only influence the customs clearance but also may cause serious penalties. In one market, the DEA in the US treats tablet presses as controlled items because they can be misused to make illegal pills. In the other, there is no single regulatory law. But a mix of GMP rules, customs checks, and criminal regulations decides what is allowed.
Many importers think these machines are just normal industrial equipment, until their shipment gets delayed or seized. So, a clear understanding of how both systems work is needed. It helps avoid trouble at customs and keeps your equipment smooth and safe.
Important notice for tablet press and capsule filling machine compliance in the US
Whatever you want to import, export, or transact a regulated machine in the US, it is a must to submit the Form 452. If you don’t have an account, create an account through DEA’s secure network system. The registration requires information such as company, address, city, state, postal code, etc. Then log in and complete the form according to the requirements.
When you handle the Form 452, it’s better to keep in mind the following details.
- Advance notice to the DEA. If you plan to import or export, submit the DEA Form 452 at least 15 days before the machines arrive at the port.
- Record keeping. You need to keep all transaction records, identities of buyers and sellers, and machine details like model and serial number. They should be kept for at least two years for DEA inspection.
- Separate reporting for each shipment. Every independent shipment must be reported individually. Even if it is the same shipper, each batch requires a separate submission.
- Return information. The importer must submit a second report via DEA Form 452 within 30 calendar days after actual receipt. The report should include the actual quantity released, the actual date released by customs, the final destination, etc.
What is the DEA and CSA in the US?
DEA stands for the Drug Enforcement Administration. It is a federal agency under the U.S.Department of Justice. It is responsible to enforce the Controlled Substances Act (CSA). Their aim is to control substances that can cause abuse, such as prescription opioids, heroin, cocaine, methamphetamine, fentanyl, etc. The DEA also works with state and local authorities to stop illegal drug production and distribution across the country.
The key content of CSA
The CSA is the main U.S. law that governs drugs, narcotics, psychotropic substances, and the equipment used to make them. Its overall structure starts with
§§801–830. The general provisions.
This part explains the purpose of the law, its scope, and the key definitions, such as what counts as a controlled substance, what “manufacture” means, and what “dispense” means.
§§821–832. Registration, records, and reporting.
This part explains who is allowed to manufacture, import, export, distribute, or possess a certain drug. It also explains what licenses are required, including DEA registration, and how to report equipment or chemicals to the DEA.
§§841–865. Criminal penalties.
These sections cover criminal penalties for illegal activities such as manufacturing, distributing, or possessing drugs, counterfeit medicines, prohibited equipment, or controlled chemicals.
These sections cover the DEA’s enforcement powers, international cooperation, and the regulatory system for imports and exports. It also includes the framework for managing regulated machines.
In this Act, there is a very important section that divides drugs into Schedule I–V (five levels). These five levels determine how risky the drug is, whether it has any legal medical use, and whether it can be prescribed.
| Schedule | Abuse Risk | Medical Use | Examples |
| Schedule I | Very high | No accepted medical use | Heroin, LSD, MDMA |
| Schedule II | High | Limited medical use | Morphine, cocaine, fentanyl, amphetamine |
| Schedule III | Medium | Accepted medical use | Certain painkillers, anabolic steroids |
| Schedule IV | Low | Accepted medical use | Diazepam, lorazepam |
| Schedule V | lowest | Accepted medical use | Certain cough preparations |
How does CSA affect your pharma equipment?
According to the CSA, anyone who manufactures, distributes, imports, or exports the following equipment must register with the DEA and report each transaction.
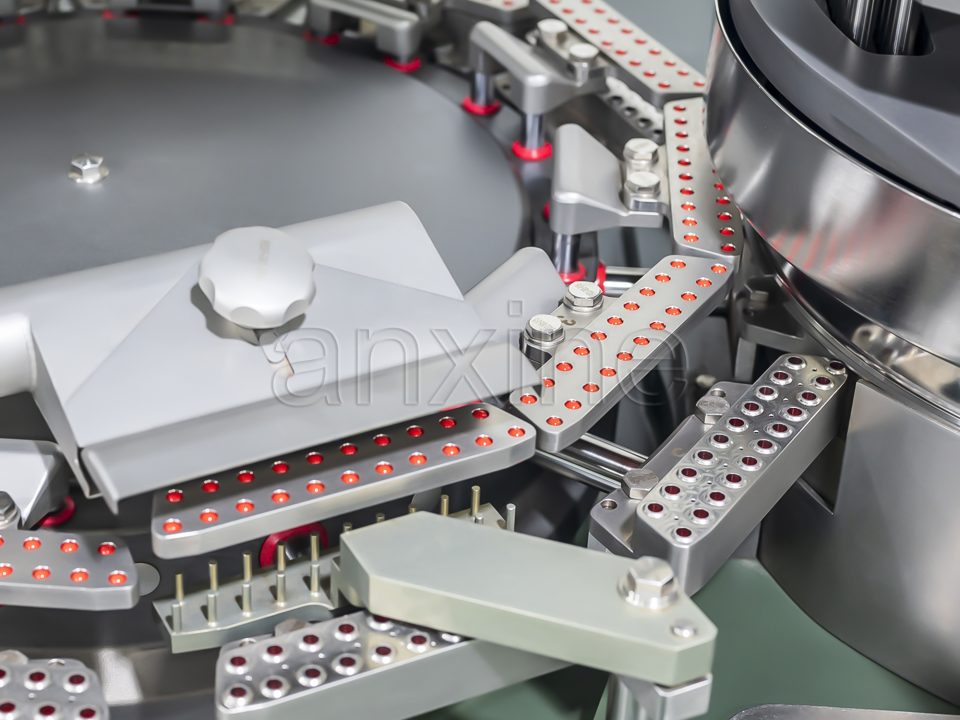
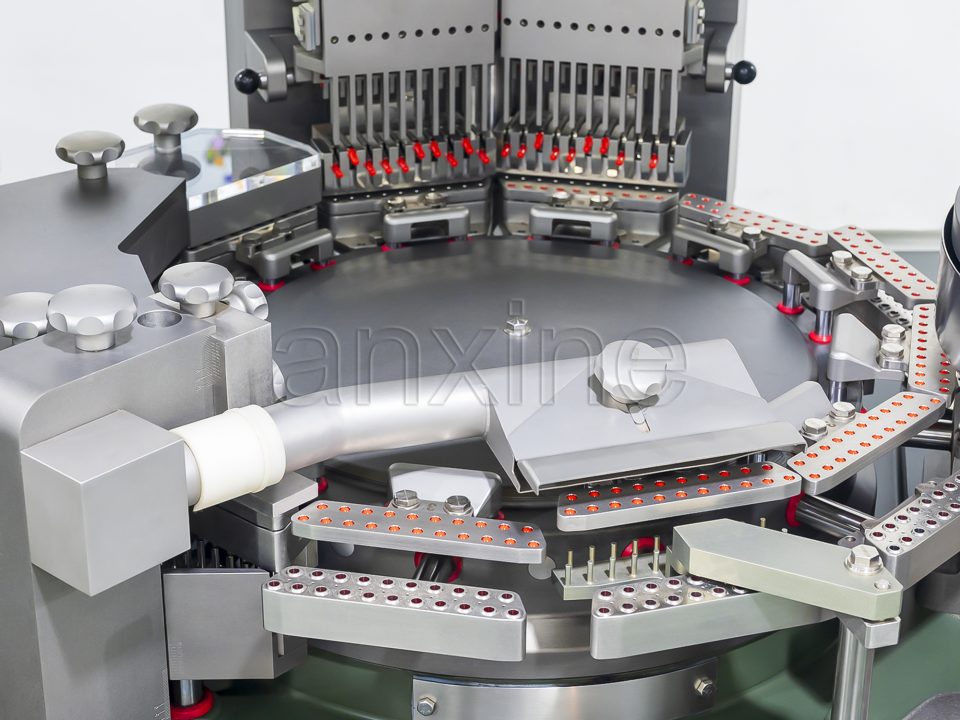
Tablet presses
A pill tablet press, including punches and dies, is regulated by the DEA. They involve single-punch models, rotary models, small lab presses, and large production presses. Any machine that can produce tablets in a consistent shape and size falls into this group.
Encapsulating machines
A capsule filling machine places powders, pellets, or liquids into hard capsules. The group covers manual, semi-automatic, and automatic machines. They are controlled because people can use them to make illegal capsules that contain controlled substances.
These machines can be used in legal pharmaceutical and supplement production. However, they may also be misused to create illegal pills. Because of this risk, the DEA monitors how these machines are traded, imported, and sold inside the United States. This helps protect public safety and reduces the chance that legal manufacturing equipment is used for harmful purposes.
What problems may occur if you don’t report or cheat the DEA?
Delays and seizure at customs
If you don’t report it, the customs can delay the shipment until the required documents are provided. They need to confirm the source, intended use, and the buyer. This process can take from a few days to several weeks. In serious cases, the equipment can be seized. These issues often lead to fines, extra inspections, and legal risk for both the buyer and the seller.
Criminal penalties
If you report it normally but actually for illegal production, you may face serious criminal penalties.
| Section | Content | Penalty |
| §841 | Illegal manufacture, distribution, or possession of controlled substances | Up to life imprisonment; fines up to 10 million USD |
| §843(a)(6) | Possessing or making equipment used to produce illegal drugs | Up to 250,000 USD in fines; up to 4 years imprisonment |
| §846 | Conspiracy, such as purchase or technical support | Same penalties as the related offense |
| §881 | Equipment and assets subject to DEA seizure | Includes machinery, raw materials, and transport tools |
Examples
Since 2019, DEA and partner agencies have reported thousands of pill presses or press-parts seizures across the US. In one recent DEA public report, the agency seized many pill presses used by traffickers to press fentanyl and other illicit drugs into fake pills. It causes some exporters to be placed on the DEA’s watchlist.
In another case in 2019, a Chinese company sold tablet presses to a U.S. private buyer without reporting the transaction. And the machines were used to produce fentanyl tablets. Both the seller and the buyer were prosecuted.
Control and supervise tablet press and capsule fillers in the EU
The EU does not regulate equipment such as tablet presses and capsule filling machines under a single law. Instead, regulation is spread across multiple areas, including pharmaceutical manufacturing, anti-drug measures, machinery safety, and export controls. They work together to regulate safety, drug-production risks, criminal misuse, and international trade.
EU regulatory framework
| Regulatory area | Regulations related to tablet press and capsule fillers | Requirements | Who is responsible | Purpose |
| Pharmaceutical manufacturing & equipment quality | EU GMP | Equipment must meet validation, cleaning, and safety requirements. | EMA + national drug authorities of EU member countries | It ensures reliable tablet production and smooth compliance review. |
| Drug-control regulation | EC No.273/2004 | Tablet presses are not controlled chemicals, but they may be considered auxiliary drug-manufacturing equipment. | EU DG HOME + national anti-drug agencies of EU member countries | If it is used to manufacture narcotics, criminal liability applies. |
| Anti-money laundering & criminal cooperation | Directive (EU) 2017/210 and Council Framework Decision 2004/757/JHA | Manufacturing, importing, or possessing equipment used to produce narcotics is illegal. | National justice and police authorities in the EU | Manufacturing, importing, or possessing equipment for producing narcotics or related tools constitutes a criminal offense. |
| Customs & export control | Regulation (EU) 2021/821 | European Commission and customs authorities | It aims at the supervision of equipment export, transfer, and cross-border movement. |
Cases
The Dutch illegal pill factory case in 2020. Police raided an illegal MDMA factory that used tablet presses manufactured in Germany. The equipment supplier was not involved in the crime. But he was warned and required to improve its KYC procedures for failing to properly vet its customers.
In Spain in 2022, police seized an illegal fentanyl tablet production line. The equipment was purchased from Eastern Europe through agents. EU law enforcement agencies warned member states to strengthen export controls on such equipment.
Main differences between the US and EU regulations to tablet press and capsule fillers
| Item | USA | EU | Differences |
| Legal form | Federal law explicitly lists tablet presses as controlled equipment. | No specific law, and criminal law applies indirectly. | USA is stricter with direct control. |
| Regulatory authority | DEA | Member states’ drug regulators, customs, and police. | EU management is decentralized. |
| Sales requirements | DEA registration and reporting required. | No registration system, but CE marking and KYC are needed. | EU emphasizes compliance checks. |
| Key risk focus | The equipment itself is controlled. | Risk depends on intended use. | EU focuses on usage intent. |
How does it affect your equipment trade with suppliers outside the EU?
At first, you need to make sure the equipment your supplier offers has already obtained CE certification. It also meets mechanical and electrical safety standards. Besides, you should clearly specify its legal pharmaceutical use in the sales contract to assure your suppliers.
On the other hand, suppliers would
- Verify the intended use of the equipment and the end user. They want to prevent its use in illegal drug production.
- Collect declarations from the end users.
- Conduct KYC to prevent resale to illegal pharmaceutical manufacturers.
- Keep sales and logistics records for at least 5 years.
- Report any suspicious use proactively to the domestic commerce or public security authorities.
How about DEA rules for your transactions with suppliers outside the USA?
Since tablet presses and capsule fillers are considered controlled equipment under U.S. law, foreign machine suppliers are careful when choosing their partners. During transactions, they generally
- Verify the buyer’s DEA registration number and collect an end-user declaration.
- Specify the legal use in the contract and keep traceable records.
- Conduct KYC (know your customer) to prevent transactions with high-risk clients.
- Retain the end-user use declaration, sales contract, and export customs declaration.
If you can provide all this information to your supplier, the trade can usually go smoothly. Please note that if your supplier finds that the equipment has an unclear destination, abnormal use, or it is suspected of drug production, they will proactively report it to the local authorities.
How to obtain a DEA license?
If you import a capsule filler from China to the US, the following details for submission of the Form 452 could be for your reference. You can click on this website and follow the steps below to complete the process.
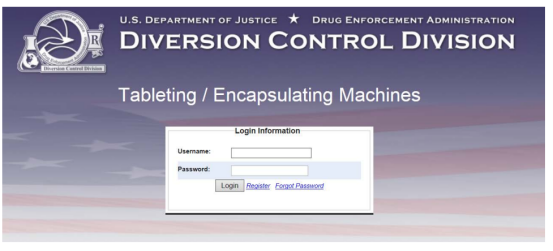
Step 1 Access the Regulated Machines Page
When you enter in, you’ll find the regulated machines home page. Then click 452.
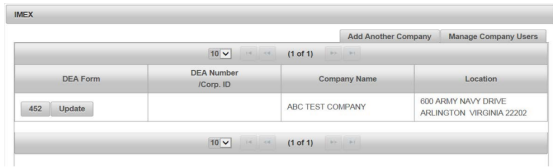
Step 2 Select Your Transaction Type
Next, indicate your transaction type.
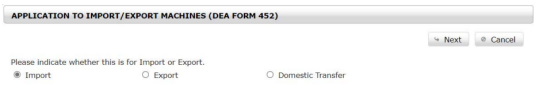
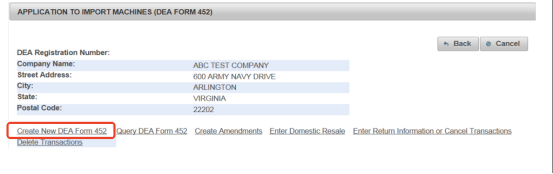
Step 3 Create a New DEA Form 452
Then, create a new DEA Form 452 for smooth import.
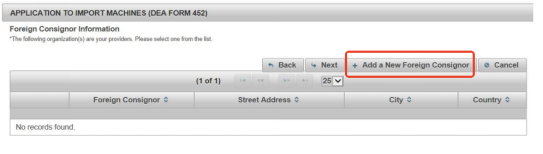
Step 4 Add a New Foreign Consignor
Next, add a new foreign consignor as required. DEA needs to know who you purchased the machine from and who is shipping it into the US. For example, you directly buy a capsule filler from Anxine. So Anxine is your supplier and its information needs to be submitted.
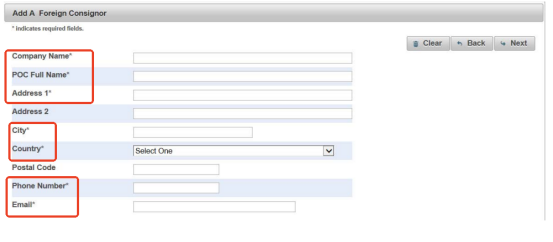
Step 5 Fill in Your Supplier’s Information
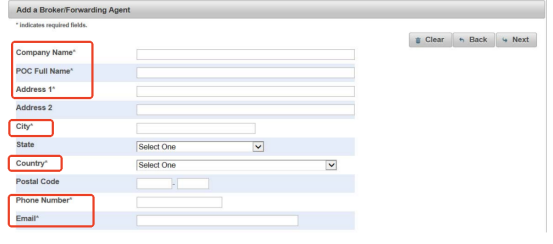
Complete the information of your supplier as required. It must have
- Company name
- POC full name
- Address
- City
- Country
- Phone number
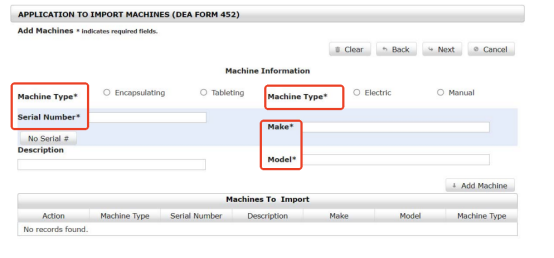
Step 6 Enter the Details of the Imported Machine
If you have finished the step above, click next. It is time to enter the machine details to the DEA Form 452. The information of your imported equipment must have
- Purpose, like pill filling or tableting.
- Machine model, electric or manual ones.
- Machine serial number.
- Make. It means your supplier name or brand. For example, Make: Anxine.
- Equipment model.
Please note that one regulated transaction can include multiple machines in different models. You only need to repeat the step to add machines to the DEA Form 452 until all your imported models are included.
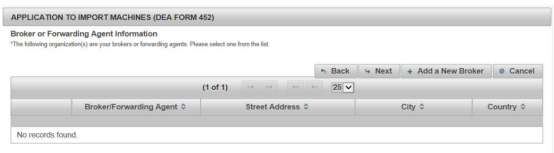
Step 7 Add Your Customs Broker
Then, add your broker. If you find an agent to help with customs clearance or shipping, their information is also required. The requirements are similar to those of your supplier.
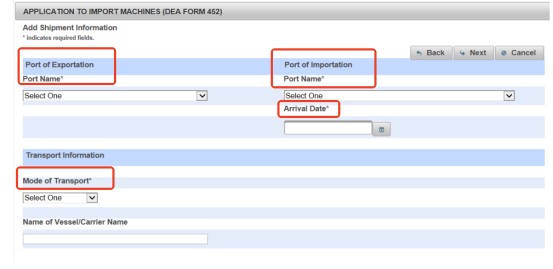
Step 8 Provide Shipping Information
Next, add your shipping information. It should include
- Port of entry
- Destination port
- Expected arrival date
- Shipping method, like sea or air shipping.
Step 9 Select the Purpose of Import
Next, the most key step comes. It is highly related to whether your machine can go to the US smoothly. Select your purpose of the machine import. Most importers choose the commercial use.
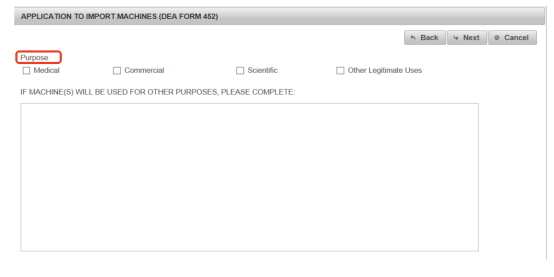
Step 10 Review and Submit Your Application
At last, review your completed application of Form 452. If no problem, submit the form to the DEA. The sample is as follows.
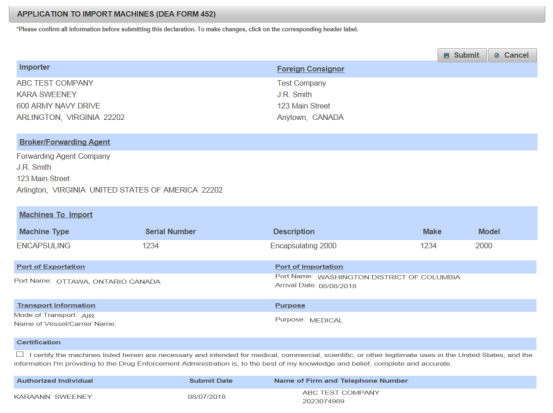
When your submission is approved by the DEA, you will receive a Transaction ID. U.S. Customs will only release your shipment after verifying this ID.