
Pressing pills can be challenging for new pharma and nutra producers. If you’re among them and struggling with tablets that crack, crumble, or vary in weight, no worries. The good news is that with the right process and equipment, you can consistently turn powders into high-quality tablets.
This guide explains how to make tablets from powder, step by step. You’ll learn what materials and tools are needed, how to use different machines to press pills, what to check after compression, and how to fix common tablet defects without guesswork.
What You Need to Prepare Before Pressing Pills
Tablet manufacturing can be simplified flow looks like this:
1. Weigh and blend the active ingredient with excipients
2. Granulate and then mill to a suitable size
3. Final blend with lubricants and glidants
4. Compress on a tablet press machine
5. Dedust and coat tablets
6. Final inspection
So, before you learn how to press pills, take a look at whether you get those essentials right.
Active Ingredient and Excipients
To make tablets from powder, your blend must do two things: fill the die consistently and bond under pressure.

Active pharmaceutical ingredient (API) delivers the tablet’s effect. Some APIs compress easily. Others are poorly flowing or moisture-sensitive. The properties can trigger capping or sticking. So the API is rarely compressed alone. It needs to be blended with excipients.
Excipients are inactive ingredients added to help form a stable tablet. The most common roles are:
- Fillers to add bulk and improve flow
- Binders to strengthen the compact
- Disintegrants to help the tablet break apart after swallowing
- Glidants to improve flow into the die
- Lubricants to reduce sticking and capping
Picking the Process Route
In the wider tablet manufacturing process, most use one of these routes:
Direct compression: This is the simplest method. You just need to blend the prepared API and excipients directly and press. But it demands that your formulation has good flow and compresses well.
Steps: Weighing/Sifting → Blending → Lubrication → Compression
Wet granulation: You mix powders and add a liquid binder to form a wet mass. Then dry and mill into granules. This method keeps ingredients uniform and together.
Steps: Weighing/Sifting → Blending → Binding → Drying → Milling/Sizing → Lubrication → Compression

Dry granulation: You use a roller compactor to compress the powder blend into slugs. Then mill these into granules. If you’re handling sensitive APIs, this approach works well.
Steps: Weighing/Sifting → Blending → Slugging → Milling/Sizing → Lubrication → Compression
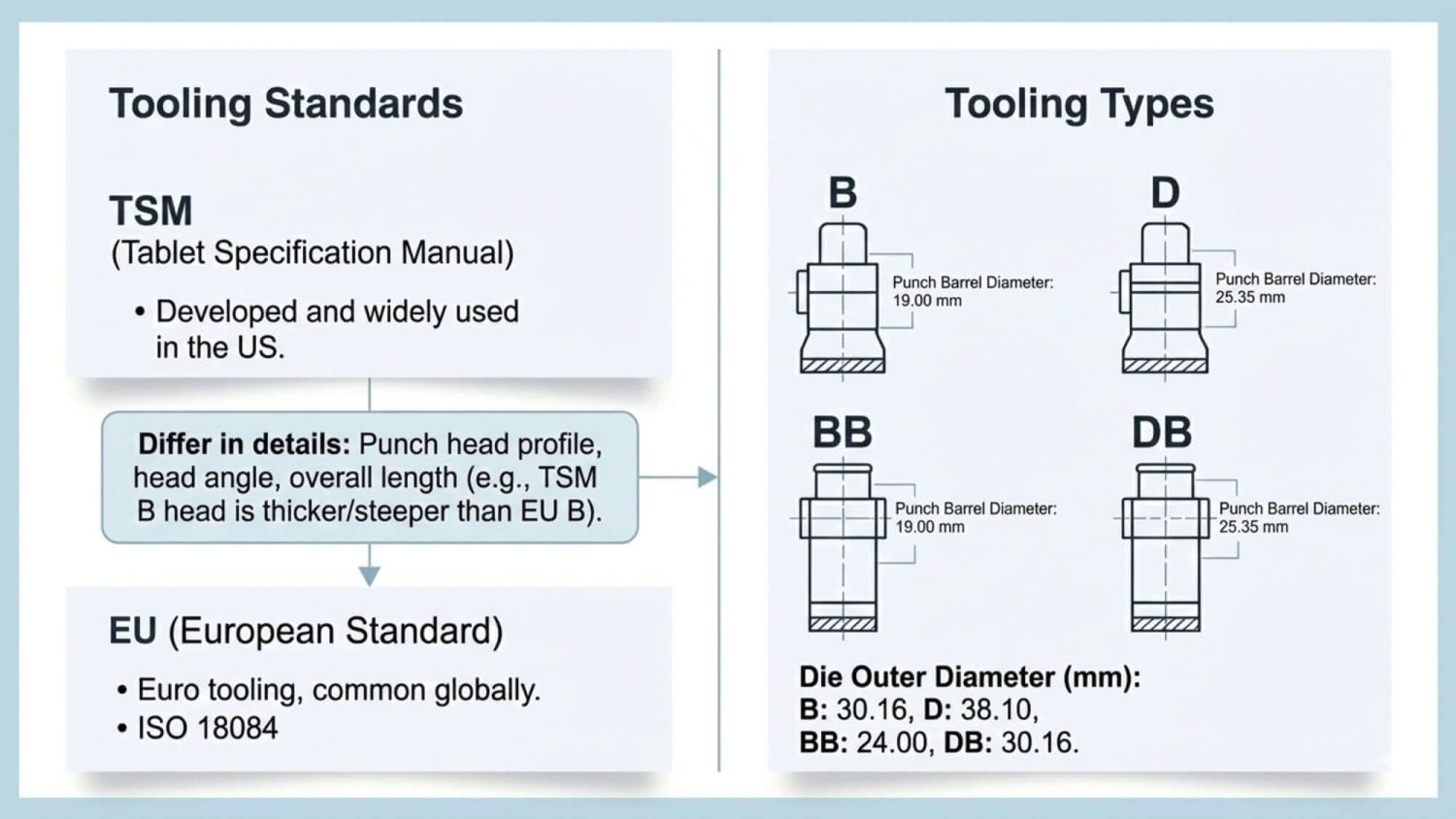
Tablet Tooling
To press pills, you need at least a set of tooling. They’re metal molds that shape and size the tablets you’re making. Each set includes an upper punch, a lower punch, and a die. Tablet Press Tooling must match the standard your tablet press is designed for. See the following infographic for common tooling standards and tooling types:
Tablet Press Machines
This is where you convert powder or granules into solid tablets. These tablet press machines can be classified into two main types:
1. Single punch tablet press: This press uses one punch and die set to produce one tablet at a time. It is usually used for clinical batches, R&D work, or small-scale production.

2. Rotary tablet press: If you look for higher output, a rotary tablet press is preferred. It uses a rotating turret with multiple sets of tooling to make pills at high speed. Compared to single-punch presses, this machine generally yields higher-quality tablets.
How to Press Pills on a Tablet Press
I’m not sure what type of press you’re using, so let’s start with a simple, easy-to-understand example: how to make tablets on a single-punch press.
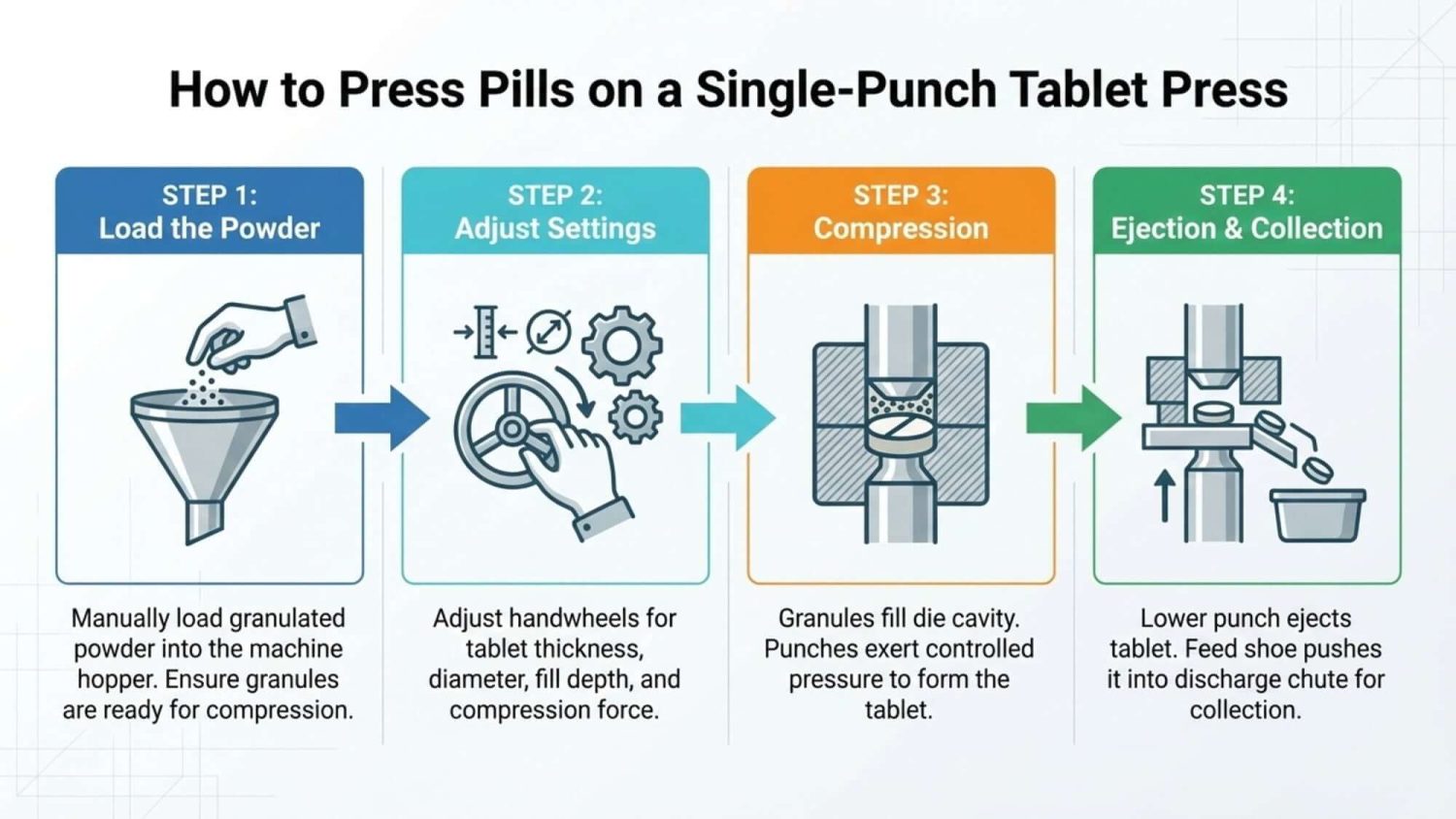
A single-punch press is great for learning the basics, but if you’re looking at real production, the rotary tablet press is what everyone actually uses. It takes many of the same steps we just talked about and speeds everything up to a whole new level. Here is how that process works.
Step 1: Feeding the Press
The hopper feeds the prepared powder or granules into a feed frame. This component is a force feeder that often comes with rotating paddles. They can spread the powder over the rotating turret.
Step 2: Filling the Dies
As the turret spins, the lower punches are pulled down by a cam track to their lowest position. This creates a cavity in the die. When each die cavity passes under the feed frame, it fills with powder. The high-speed paddles inside the frame ensure the powder flows consistently.
Step 3: Metering the Dose
This step is to ensure every pill has the exact amount of API. After filling the powder, the die hole might not get the same amount of powder. So the machine moves up the lower punch slightly over a dosing cam.
This movement pushes out a tiny bit of excess powder. And then the powder is scraped away by a blade. The powder left in the die cavity is the final dose of the tablet.
Step 4: Tablet Compression
Now the real pressing happens. The loaded dies travel under a set of compression rollers. Rotary presses usually compress the powder twice.
1. Pre-compression: The punches pass between two small rolls that apply light pressure. This makes the punches squeeze gently and compact the powder into a soft slug. This step squeezes the air out of the powder.
2. Main compression: Immediately after, the turret brings the same tooling under the huge main rolls. Here, the upper and lower punches press together at a massive force to bond the powder firmly into a solid pill.
Step 5: Ejection of Tablets
After compression, the turret continues rotating. The upper punches are pulled high up by a cam mechanism. At the same time, the lower punches ride up a slightly steep cam track. This pushes the tablet upward out of the die hole. A take-off bar then guides the tablet onto a chute or into a collection bin.
Key Factors for Pressing Consistent Pills
Formulation
How well the particles in your formulation flow, compress, and are distributed all affect tablet formation. If the powder clumps, the die hole won’t fill completely. If your mix is loose, the tablet is more likely to crumble.
Compression Force
If the pressure is too low, your tablet will be soft and prone to breaking. Too much, and the pills probably end up with capping or lamination. The tooling can even be damaged.

Dwell Time
Dwell time is how long the punches stay in contact with the compression rolls at full force. The duration determines if the air has enough time to escape. If the time is too short, tablets may not bond well. This can be another cause of capping.
Tablet Press and Tooling Condition
Your tablets are only as good as your compression machine, punches, and dies. Damaged cams and rolls, or poor lubrication, can cause inconsistent force. Worn die cavities give you ridged tablets. Scratched or pitted punch faces will cause the powder to stick to the tooling.

Environmental Factors
Both the room and the machine can heat up during long runs. If temperature or humidity changes, your powder may compress differently. For example, high humidity can make the powder sticky. And if the room is too dry, static electricity can build up. Then the powder may fly around or cling to the machine parts.
What to Check After Tablet Compression
After you press tablets, you still need to confirm they meet your quality specs. Here are the key quality checks to help you catch issues early.
- Weight: Individual tablets are usually allowed to vary by ±10%. Based on the average tablet weight, the limit may tighten to ±7.5% or ±5%. When you test 20 tablets, no more than 2 can fall outside the limit. And none can be outside by more than 2× the stated percentage.
- Hardness: Use a tablet hardness tester to measure how much force (in N or kp) it takes to break a tablet. Tablets need to be strong enough for coating, packaging, and transport. But they can’t be too hard, or they may dissolve too slowly. Many standard tablets are in the 40-80 N range.
- Thickness and Diameter: If thickness is drifting, you’ll see problems like weight variation and poor packaging fit. Thickness should be consistent. Diameter should match your punch design. A simple calliper check can confirm tablet dimensions.
- Friability: Run a friability test to check whether tablets crumble or shed too much powder. Tumble 20 tablets in a rotating drum for 100 revolutions, then measure the weight loss. A common target is about 0.5%-1.0% weight loss or less.
- Visual Inspection: Check tablets for visible flaws like cracks, chips, or lamination. Every tablet should look smooth and intact. You can use the tablet and capsule inspection systems we mentioned above. If you spot defects, keep reading. The next section will explain the likely causes and fixes.
Common Pill Pressing Issues and Solutions
| Defect | What You See | Likely Causes | Practical Fixes |
|---|---|---|---|
| Capping | Tabel top or bottom separates. | Trapped air; weak bonding; granule too dry or brittle. | Add pre-compression; increase dwell time; adjust moisture or binder. |
| Lamination | Tablet splits into horizontal layers. | Weak bonding; trapped air; high speed. | Change binder; increase pre-compression; slow down press speed; adjust main force profile. |
| Sticking | Powder sticks to punch faces or die walls. | Too much moisture; not enough lubricant; rough tooling. | Control moisture; adjust lubricant level; polish punches. |
| Cracking | Small visible cracks on tablet surfaces. | Too much compression force; brittle formulation. | Reduce main compression force; add a plasticizer; increase moisture in granules. |
| Chipping | Tablet edges break. | Brittle granules; worn dies; too much ejection stress. | Change the process route; replace damaged tooling; improve lubrication. |
| Weight variation | Tablets are too light or too heavy. | Uneven die filling; poor powder flow; machine vibration. | Add glidant; use a force feeder; slow turret speed. |
| Hardness issues | Tablets break easily or feel like a stone. | Too low or too much compression force; unbalanced formulation. | Adjust force; optimize formulation |
Key Compliance Requirements for Tablet Compression Process
To manufacture tablets for the U.S. market, you must comply with FDA cGMP regulations (21 CFR Parts 210, 211, or 111 for supplements) to ensure product safety, identity, and purity.
| Focus Area | Core Requirements |
|---|---|
| Quality management | Follow Standard Operating Procedures (SOPs) for every step and maintain detailed batch records for full traceability. |
| Equipment | Ensure all machinery is qualified, calibrated, and suitable for its intended use. |
| Rigorous testing | Use in-process controls and final quality tests (weight, hardness, friability, etc.) to ensure tablets meet all standards. |
| Environment | Maintain sanitary conditions; use stainless steel contact parts and control temperature and humidity to prevent contamination. |
| Operator | Staff must be properly trained in hygiene, safety, and technical tasks to prevent production errors. |
Final Thoughts on How to Press Pills
Pressing pills is not just about running a tablet press. It is about understanding how your formulation, tooling, machine settings, and environment all work together.
If you:
- Prepare blends that flow and compress well
- Choose and maintain the right tablet press and tooling
- Follow clear tablet compression steps
- Monitor the process and fix issues fast
- Work within cGMP and keep solid documentation
Then you will find that pressing pills becomes a controlled, repeatable process.



